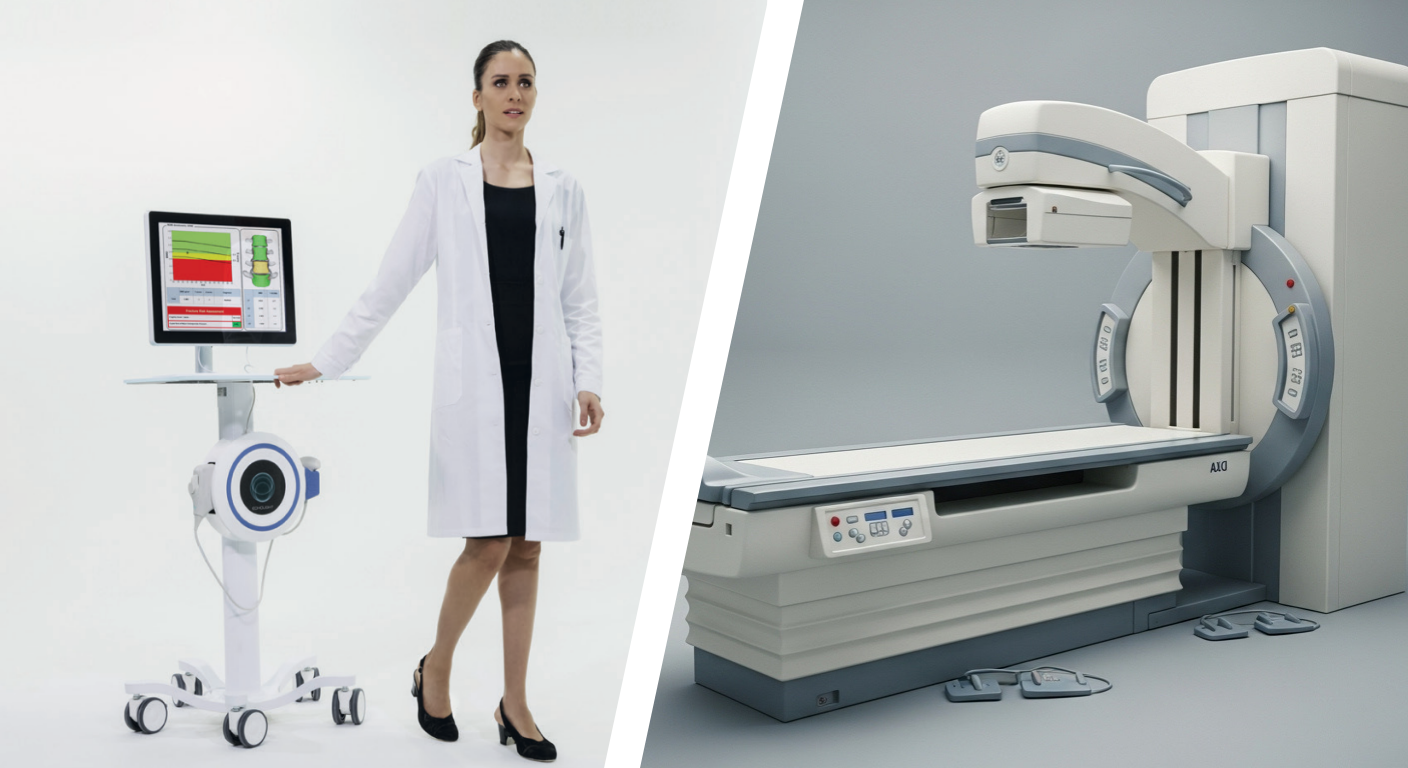
Accurately assessing a patient’s fracture risk is a cornerstone of proactive musculoskeletal health management. For decades, Dual-Energy X-ray Absorptiometry (DXA, or DEXA) has been the gold standard for measuring Bone Mineral Density (BMD). However, its reliance on ionising radiation and specific infrastructure requirements present known limitations – including the need for dedicated space, high capital costs, and lack of portability.
Today, a novel approach is reshaping the landscape of osteoporosis diagnostics. Radiofrequency Echographic Multi Spectrometry (REMS) is an innovative, radiation-free technology that offers a comprehensive assessment of bone health. This article provides a direct clinical overview of the REMS technology, comparing it head-to-head with DXA to help clinicians evaluate its role in modern practice.
Radiofrequency Echographic Multi Spectrometry (REMS) is a non-ionising diagnostic technology used to assess bone health at key axial sites — specifically the lumbar spine and femoral neck. Its primary purpose is to deliver a complete diagnostic report, including Bone Mineral Density (BMD), T-scores, and Z-scores, without exposing patients to radiation.
Developed in Italy and validated through extensive clinical trials, REMS provides a robust method for diagnosing osteoporosis and monitoring changes in bone mass over time. It has also been evaluated and endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) as a valuable tool for bone health assessment.
The REMS technique uses a standard convex ultrasound probe to scan the lumbar spine and femoral neck — the same gold standard sites used in DXA for diagnosing osteoporosis, as recommended in the Malaysian Clinical Practice Guidelines. During the scan, which lasts only a few minutes, the system acquires thousands of raw, unfiltered radiofrequency signals.
An advanced algorithm then processes these signals, comparing the patient’s unique bone “signature” against reference spectral models from a vast database. This analysis yields the quantitative data—BMD, T-score, and Z-score — that clinicians rely on for diagnosis according to World Health Organisation (WHO) guidelines.
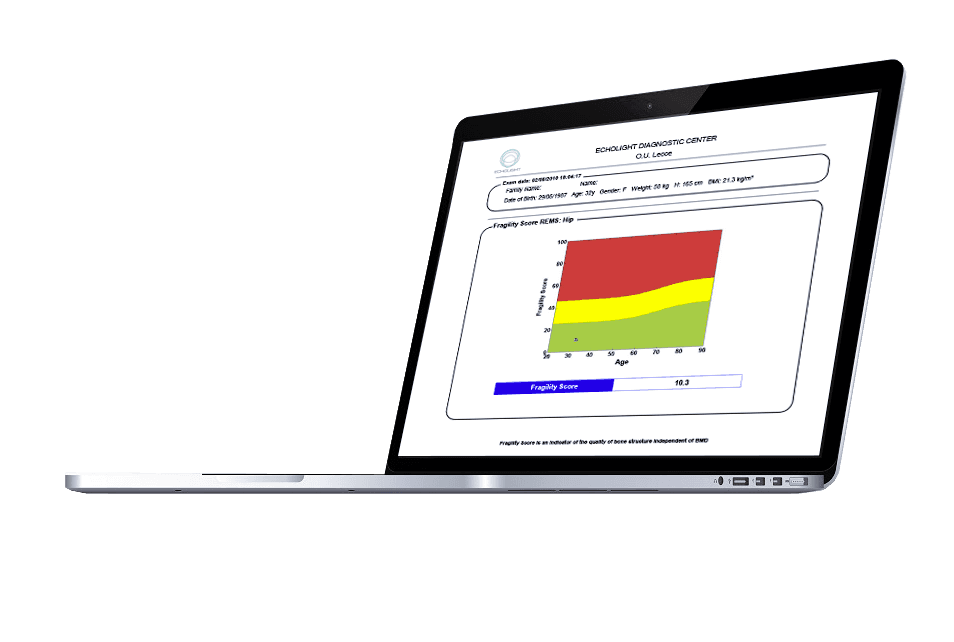
A key innovation of REMS technology is its ability to assess bone quality, a critical factor in fracture risk that BMD alone does not capture. By analysing the micro-architectural characteristics of the bone from the ultrasound signals, REMS calculates a proprietary Fragility Score.
This score is a parameter, independent of BMD, that quantifies bone’s resistance to fracture. Recent studies have demonstrated that the Fragility Score is a powerful predictor of incident fragility fractures over a five-year period, providing clinicians with a more complete risk assessment. Notably, this five-year fracture risk prediction has been shown to closely correlate with the 10-year FRAX fracture risk derived from DXA, reinforcing its clinical relevance and reliability.
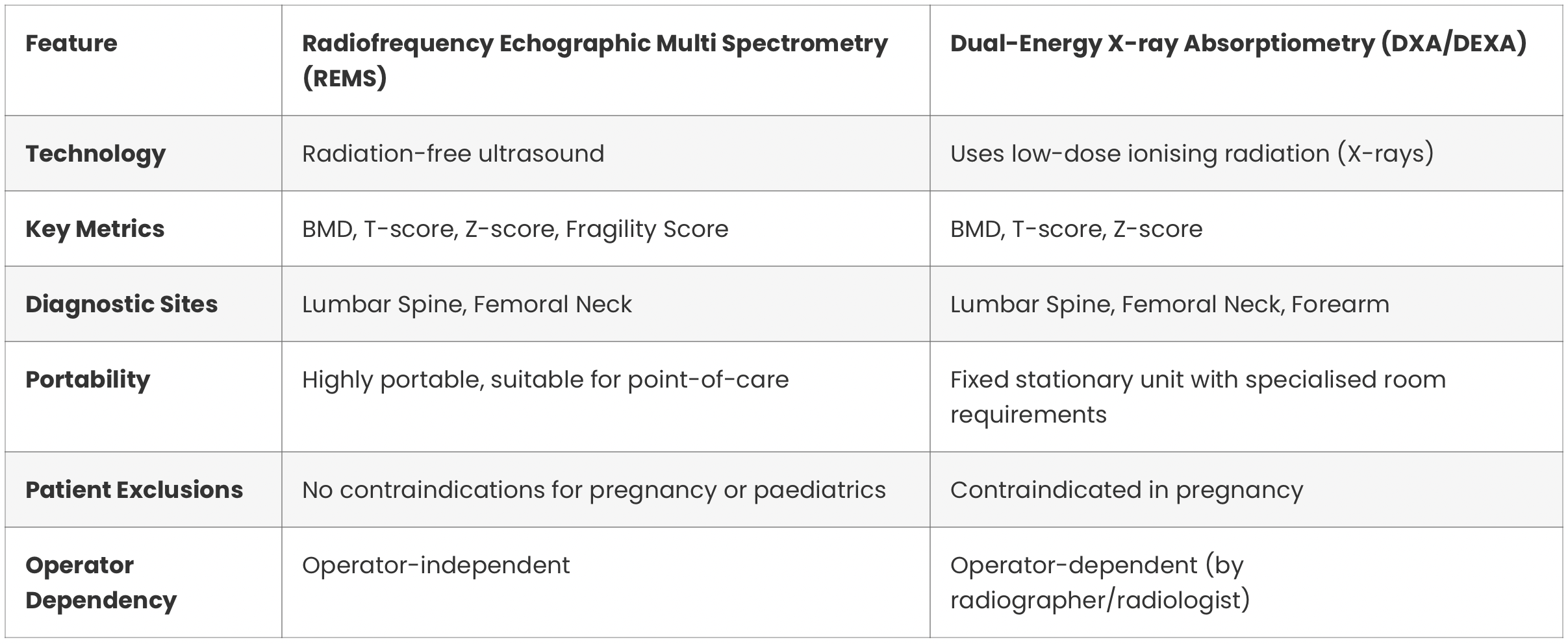
For clinicians familiar with DXA, understanding the practical differences and advantages of REMS is essential. The following table provides a clear comparison of the two technologies.

The REMS approach offers several distinct clinical advantages. Its radiation-free nature makes it an ideal diagnostic tool for radiation-sensitive populations, including paediatric patients, pregnant women, and patients requiring frequent follow-up scans.
Furthermore, the high portability of REMS devices allows for diagnostics at the point of care, such as in a private clinic, at the bedside, or in remote community settings. This technology is also particularly valuable for assessing patients with spinal deformities (e.g., scoliosis), vertebral fractures, metallic implants, osteophytes, aortic calcification, or other degenerative changes, as these conditions can create artefacts that may render DXA results inconclusive

Yes, the technology behind REMS has received clearance from the U.S. Food and Drug Administration (FDA) and is also CE-marked in Europe. This regulatory approval signifies that the technology has been deemed safe and effective for its intended use in clinical practice.
The FDA clearance, coupled with a growing body of peer-reviewed research published in journals like Bone and the Journal of Bone and Mineral Research, establishes REMS as a credible and validated diagnostic tool.
While REMS is suitable for the general population, it offers unique benefits for specific patient groups where DXA may be limited or contraindicated.
Radiofrequency Echographic Multi Spectrometry (REMS) represents a significant evolution in osteoporosis diagnostics. It is a clinically validated, FDA-cleared, and radiation-free tool that provides data highly concordant with the long-standing DXA gold standard.
By offering a more complete assessment of fracture risk through its unique Fragility Score, REMS empowers clinicians to make more informed decisions. Its safety, portability, and accuracy in complex patient cases position it as a powerful tool that can complement—and in many situations, enhance—the current standard of care.
For a deeper understanding of how REMS can be integrated into your practice, contact Nuvera for a demo today.
The primary difference is the core technology. REMS uses radiation-free ultrasound waves to assess bone health, while DXA uses low-dose X-rays (ionising radiation). A significant advantage of REMS is its additional ability to evaluate bone quality via the Fragility Score, providing a more comprehensive fracture risk assessment.
Yes. Because the REMS scan is based on ultrasound technology, it is free from ionising radiation. This makes it exceptionally safe for all patients, including pregnant women, children, and individuals requiring frequent monitoring where repeated exposure to X-rays would be a concern.
Multiple international, multi-centre studies have shown that REMS delivers BMD measurements with high diagnostic concordance and strong correlation to DXA. REMS has proven highly accurate for diagnosing osteoporosis based on WHO criteria, with some studies reporting a lower margin of error for repeat scans. Sensitivity and specificity exceed 90%, and a strong correlation was observed between REMS and DXA BMD values, particularly at the lumbar spine (r = 0.94, p < 0.001) and femoral neck (r = 0.93, p < 0.001).
No, unlike DXA machines which typically require daily calibration, the REMS machine only requires calibration once every 6 months.
2025 © Nuvera